
Eko Health operates in the medical technology space, focusing on digital offerings designed to support heart and lung assessment in clinical settings.
The brand offers digital stethoscopes and software-based resources that pair enhanced audio, visual waveforms, ECG capabilities, and artificial intelligence features.
These offerings are intended for use by healthcare professionals across individual practices, health systems, and life science applications. They claim to target challenges related to the early detection of cardiovascular and pulmonary conditions.
In this review, we examine the brand’s core background, product ecosystem, clinical use cases, and regulatory positioning. The review also discusses the associated advantages and potential limitations of the brand.
Founded in 2013, Eko Health offers digital auscultation products intended to support earlier detection of heart and lung conditions during routine clinical exams.
The brand focuses on modernizing the traditional stethoscope by combining enhanced audio hardware with software and artificial intelligence.
Its devices amplify body sounds, reduce background noise, and convert auscultation data into visual waveforms and ECG tracings.
AI algorithms analyze heart sounds and electrocardiogram data in real time to flag clinically relevant indicators such as heart murmurs, atrial fibrillation, abnormal heart rates, and low ejection fraction.
The brand’s product portfolio includes the Eko CORE 500™ Digital Stethoscope, which functions as a digital platform that combines high-fidelity auscultation with a built-in display and 3-lead ECG capability. Its 3M™ Littmann® CORE Digital Stethoscope integrates the brand’s digital technology into a cardiology-grade Littmann stethoscope. Beyond these, the brand also offers the Eko CORE™ Digital Attachment, which is aimed at converting existing analog stethoscopes into amplified digital devices.
Use cases of the brand’s offerings cover primary care, cardiology, nursing, emergency medical services, pediatrics, veterinary medicine, telehealth, and clinical education.
According to its official website, the brand’s hardware and analysis software hold multiple FDA clearances and are certified under ISO 13485 and MDSAP standards. However, AI features are regulated for use only in regions where clearance has been granted and are intended to support, not replace, clinician judgment.
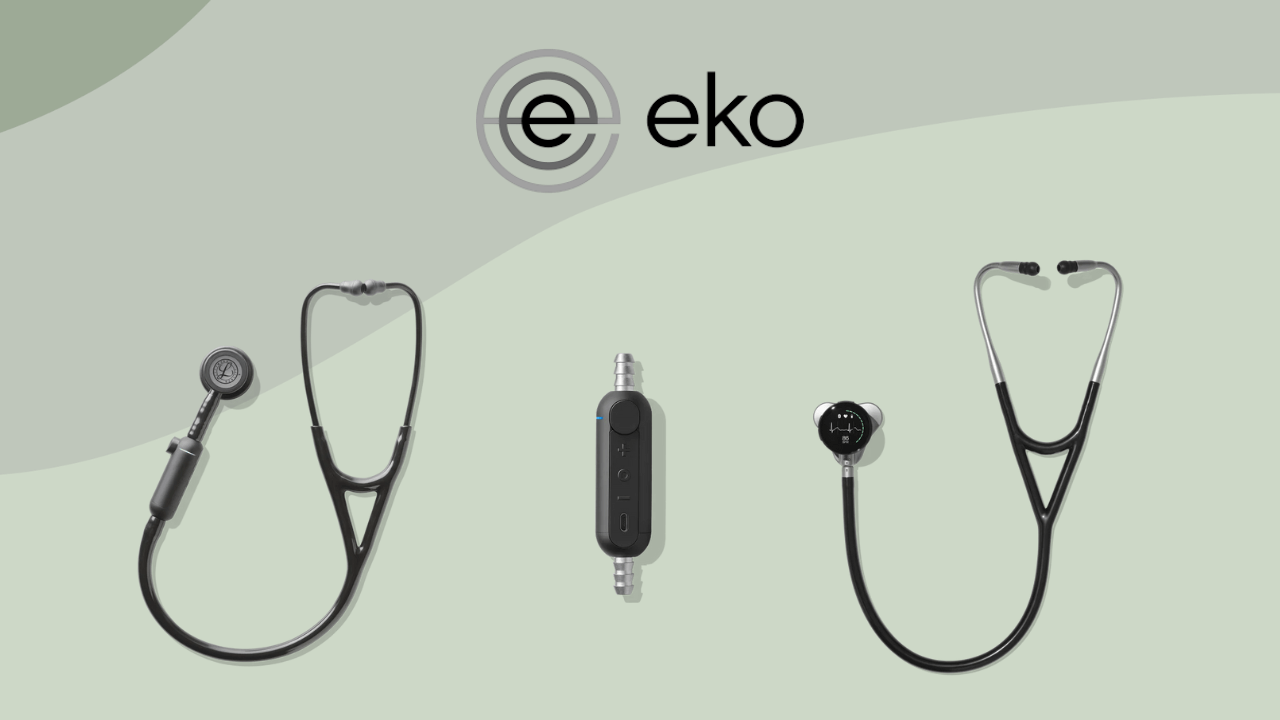
The 3M™ Littmann® CORE Digital Stethoscope integrates the established acoustic performance of 3M™ Littmann® with digital signal processing technology, creating a hybrid analog–digital instrument intended for use in modern clinical environments.
According to the official website, the stethoscope measures 27 inches (69 cm) from ear tips to chestpiece and weighs 8.1 oz (232 g), with a 3.1 oz (87 g) chestpiece. It features a double-sided chestpiece with adult and pediatric diaphragms made from epoxy/fiberglass, using a tunable single-piece design.
The binaural tubing uses a double-lumen configuration, paired with soft-sealing ear tips to improve acoustic isolation. Power is supplied by a rechargeable lithium-ion battery charged via Micro-USB, delivering up to 480 minutes of continuous digital use and roughly two weeks of typical clinical operation.
3M™ Littmann® CORE Digital Stethoscope is built on the Cardiology IV™ platform and pre-assembled with the Eko CORE™ Digital Attachment, supporting both traditional acoustic auscultation and digitally amplified listening.
The device amplifies body sounds up to 40× across seven volume levels, improving detection of low-amplitude cardiac murmurs, gallops, crackles, and subtle pulmonary sounds that might otherwise be masked by ambient noise.
Active noise cancellation may reduce external interference, while the tunable diaphragm enables selective emphasis of low- or high-frequency sounds, supporting assessment of both adult and pediatric heart and lung physiology.
When paired with the Eko App, real-time waveform visualization converts acoustic signals into visual phonocardiograms, helping clinicians correlate sound patterns with underlying cardiac valve dynamics and airflow mechanics.
Eko CORE 500™ Digital Stethoscope is positioned as a replacement for the traditional analog stethoscope, i ntegrating digital auscultation with real-time cardiac electrical data. It features advanced acoustic processing, a built-in full-color display, and an integrated 3-lead ECG to support more comprehensive cardiopulmonary assessment at the point of care. Construction includes an anodized aluminum headset, vinyl tubing, a sealed speaker system, and silicone ear tips.
Eko CORE 500™ Digital Stethoscope enhances detection of subtle heart and lung sounds through TrueSound™ technology, delivering up to 40× amplification with active noise cancellation.
Digital in-ear speakers transmit sound directly rather than relying on air-filled tubing, reducing signal loss and external interference. It supports Cardio, Pulmonary, and Wide audio filters, each tuned to different frequency ranges to isolate cardiac, respiratory, or full-spectrum body sounds.
The integrated 3-lead ECG captures electrical cardiac activity with a frequency response range of 0.1–250 Hz. Three evenly spaced electrodes within the single-sided chestpiece enable rhythm analysis and improved identification of arrhythmias when paired with waveform visualization.
A built-in full-color LCD shows 1-lead ECG data, exam details, and device settings directly on the stethoscope. This may reduce reliance on secondary screens and support faster clinical decision-making during exams.
As per its official website, the Eko CORE™ Digital Attachment intends to transform traditional (analog) stethoscopes into a digitally enhanced clinical assessment device. It attaches directly to most adult and pediatric stethoscopes, preserving familiar ergonomics while adding digital sound processing, amplification, and wireless connectivity.
The attachment is compact and lightweight, measuring 2.8 inches (71 mm) in height and 0.9 inches (23 mm) in width and depth, with a total weight of approximately 1 ounce (28 grams). It is constructed from durable polycarbonate thermoplastic and does not have natural rubber latex or phthalate plasticizers. The ability to switch between amplified listening and analog modes allows clinicians to compare traditional sound profiles with enhanced digital output during the same exam.
Eko CORE™ Digital Attachment integrates with the Eko App via Bluetooth 4.2 low-energy connectivity, enabling real-time waveform visualization, wireless listening, and single-sound recording. Digital visualization of auscultatory sounds helps t ranslate auditory findings into visual patterns, supporting teaching, documentation, and longitudinal monitoring.
Eko’s technology is reported to be trusted by large health systems and deployed within organizations such as the NHS, indicating adoption at an institutional level rather than limited individual use.
With claims like more than 650,000 devices sold globally, the brand’s footprint reflects sustained engagement with clinicians who require dependable products that align with professional standards of care.
Such partner identity is reinforced through Eko’s product strategy, which focuses on enhancing established clinical instruments instead of introducing isolated or consumer-style devices. Offerings such as the 3M™ Littmann® CORE Digital Stethoscope reflect collaboration with legacy medical equipment brands, pairing traditional acoustic performance with Eko’s digital amplification and analysis capabilities.
The CORE 500™ Digital Stethoscope further integrates digital listening, sound visualization, and algorithm-supported insights into a single clinical device, supporting use in structured care settings. For clinicians who wish to upgrade existing tools, the CORE™ Digital Attachment allows integration of digital functionality without changing established equipment preferences.
Eko Health positions itself with a global health impact orientation by focusing on technologies that are deployed across diverse healthcare systems to support earlier detection of cardiovascular and pulmonary conditions. Its devices and software have been adopted internationally, including deployments within public health systems and large provider networks, indicating applicability beyond a single market or care model. Such a global reach reflects an emphasis on scalable diagnostic support rather than region-specific approaches.
The brand’s impact strategy centers on early detection enabled through AI-supported features, which can be applied consistently in primary care, specialty clinics, and large population health initiatives. Eko has partnered with health systems, research institutions, and clinical trial programs to validate and refine its algorithms, allowing insights derived from large and diverse patient populations to inform ongoing development. These collaborations are intended to help scale the clinical usefulness of AI-driven sound analysis while maintaining alignment with established diagnostic practices.
Eko Health’s growth and market expansion are closely tied to partnerships with health systems, institutional buyers, and clinical research networks, which may shape how and where the brand’s products are adopted.
A significant portion of Eko’s sales and implementation comes through collaborations with hospitals, clinics, government health services such as the NHS, and participation in clinical trials that evaluate its digital auscultation and cardiopulmonary monitoring technologies.
As decisions in these settings are influenced by procurement budgets, institutional priorities, regulatory requirements, and trial outcomes, Eko’s ability to expand its user base can be paced by external partner timelines rather than by direct consumer demand. It holds particular relevance for products like the 3M™ Littmann® CORE Digital Stethoscope, CORE 500™ Digital Stethoscope, and the CORE™ Digital Attachment, which are often integrated into clinical workflows through organizational purchasing agreements and clinician training programs.
While these offerings are intended to deliver high-fidelity auscultation and enhanced diagnostic data, their adoption outside of partner networks depends in part on endorsement, reimbursement policy, and integration support from the health systems that deploy them.
Without broad direct-to-consumer distribution or a standalone marketing presence, prospective buyers may have more limited awareness or access to these products compared with products sold through general retail channels.
Both Thinklabs and Eko Health focus on modernizing auscultation devices and techniques, but they have fundamentally different technical and clinical philosophies. Thinklabs is rooted in audio engineering, with its origins tracing back to the early 1990s and a long-standing effort to preserve the natural sound profile clinicians are trained to recognize.
Eko Health emerged more than two decades later from cardiology workflow research, positioning auscultation as an entry point for AI-assisted disease detection and longitudinal clinical intelligence.
Thinklabs’ identity is built around its proprietary Electromagnetic Diaphragm transducer. Such technology is designed to detect diaphragm vibration through a high-intensity electric field, allowing the device to convert sound into an electrical signal without altering the acoustic character clinicians expect from traditional stethoscopes.
Eko Health’s identity is broader and more platform-oriented. Its identity centers on supporting informed clinician judgment through a combination of digital auscultation, real-time signal processing, FDA-cleared AI algorithms, and cloud-based data infrastructure. Rather than focusing exclusively on preserving traditional sound authenticity, Eko emphasizes enhanced detection, visualization, and pattern recognition, particularly for cardiovascular conditions that may be missed during routine exams.
Product structure reflects the differences between brands clearly. Thinklabs maintains a tightly focused ecosystem centered on the Thinklabs One Digital Stethoscope, which is a compact, palm-sized device delivering up to 100× amplification with five selectable filter modes optimized for cardiac, pulmonary, and general use.
Variants such as the Thinklabs One+Y bundle add a traditional binaural headset, while the Telemedicine Kit packages the device with verified USB audio interfaces, splitters, and cabling for remote auscultation.
Meanwhile, Eko Health offers a tiered hardware lineup designed to scale across different practice settings. The Eko CORE™ Digital Attachment allows clinicians to convert an existing analog stethoscope into a digital device, adding amplification, noise cancellation, and AI support when paired with Eko’s software. Its 3M™ Littmann® CORE Digital Stethoscope integrates the brand’s electronics into a cardiology-grade Littmann chassis, while the Eko CORE 500™ Digital Stethoscope combines digital auscultation with a built-in color display, in-ear speakers, and an integrated three-lead ECG.
Software and data handling represent a pronounced difference between the brands. Thinklabs intentionally avoids proprietary software layers and algorithmic interpretation, instead prioritizing compatibility with existing recording systems, telehealth platforms, and clinical workflows. Sound can be transmitted, recorded, or shared, but interpretation remains entirely clinician-driven.
Meanwhile, Eko Health positions software as a central pillar of its value proposition. Through its subscription-based Eko+ platform, clinicians gain access to FDA-cleared AI models that support the detection of heart murmurs, atrial fibrillation, tachycardia, bradycardia, and low ejection fraction, often delivering feedback within seconds during an exam. Visual phonocardiograms, optional ECG tracings, cloud storage, and longitudinal comparison tools transform auscultation into a data-rich process.
Littmann represents an auscultation philosophy grounded in decades of acoustic engineering, clinical reliability, and mechanical consistency. Operating under the Solventum portfolio, the brand has historically focused on refining sound transmission, ergonomics, and durability rather than rapidly cycling through new technologies.
Such a philosophy is reflected in Littmann’s tiered product structure. The brand offers the Classic III Stethoscope, featuring tunable diaphragms for adult and pediatric use cases, balanced acoustic sensitivity, and a lightweight build intended for extended daily wear. It also offers the Cardiology IV Stethoscope, which emphasizes passive acoustic performance in noisy clinical settings. Its larger chestpiece, deeper bell, and dual-lumen tubing are intended to reduce ambient interference and improve detection of subtle cardiac and pulmonary sounds. Littmann’s most advanced option, the Littmann CORE Digital Stethoscope, introduces digital amplification and noise cancellation while retaining a traditional cardiology-grade form factor. The device allows switching between analog and amplified listening, offering up to forty times amplification at peak frequencies. Bluetooth connectivity enables pairing with Eko software for waveform visualization, recording, and optional AI analysis.
Meanwhile, Eko Health approaches auscultation from a data-first perspective, considering body sounds as analyzable clinical signals rather than transient auditory impressions. Its ecosystem integrates digital hardware, FDA-cleared AI algorithms, real-time visualization, and cloud-based storage.
Eko’s hardware lineup scales digitally rather than acoustically. The 3M Littmann CORE Digital Stethoscope pairs Littmann’s acoustic design with Eko’s digital technology into a single instrument. The CORE Digital Attachment converts existing analog stethoscopes into digital devices by adding amplification, noise reduction, and AI support through the Eko+ platform. The brand also offers the CORE 500 Digital STethoscope, which combines high-fidelity digital auscultation with an integrated color display, in-ear speakers, and built-in three-lead ECG capability.
Software represents the primary dividing factor between the brands. Littmann positions digital functionality as an optional extension layered onto proven acoustic stethoscopes, enabling gradual adoption without disrupting established clinical workflows. Meanwhile, Eko positions software as central, with its Eko+ subscription providing access to AI-driven murmur detection, atrial fibrillation analysis, low ejection fraction screening, cloud storage, and longitudinal comparison. These capabilities are designed to integrate with electronic medical records and enterprise systems.
In evaluating the brand, we analyzed its operational background and ratings across multiple review forums, focusing specifically on brand reputation, complaint handling, and consistency of customer experience.
On the Better Business Bureau, the brand holds a D- rating, despite indicating 13 years in business. BBB records show multiple unresolved complaints centered on billing disputes, delayed or missing deliveries, warranty fulfillment issues, and the lack of follow-up from customer support. Several users described prolonged attempts to obtain refunds or replacements for the brand’s devices without resolution.
Meanwhile, TenereTeam provided the brand with a 4.8 out of 5 score based on 2,300+ ratings, supported by generally positive sentiment around product performance and technological value. However, the feedback leaned more on perceived product capability rather than post-purchase support or dispute resolution.
Many users appreciated the brand’s advanced digital stethoscope technology and AI-driven resources, although its public reputation is impacted by customer service execution, fulfillment reliability, and subscription transparency.
To evaluate the brand, we assessed its real user feedback on Reviews.io, where the brand currently has a 4.5 out of 5 rating from 400+ reviews.
Many users expressed satisfaction with the product concept and hardware performance when devices function as expected. Positive experiences often highlighted responsive, human-led customer support in specific cases, such as prompt replacements for defective units and courteous interactions with named support staff.
At the same time, a major portion of negative feedback centered on order fulfillment, billing clarity, and customer support accessibility. Some users reported canceled orders without explanation, partial or delayed refunds, and undelivered shipments. Subscription-related feedback introduced additional complexity into the evaluation.
Several reviewers describe frustration with EKO+ membership pricing, feature paywalls introduced after purchase, and confusion around what was included in the base product versus ongoing subscription fees.
Some users also expressed hardware durability concerns, such as buttons failing within months of use, and uncertainty around warranty responsibility between Eko Health and retail partners.
Our evaluation of the brand’s user feedback indicates that it maintains a strong average rating on Reviews.io, driven by satisfied customers and effective product replacements. However, ongoing concerns with transparency, subscription changes, and order fulfillment reliability indicate areas of improvement for the brand.
Eko Health centers its offerings on AI-enabled digital auscultation devices built for regulated clinical settings. FDA-cleared detection for heart murmurs, atrial fibrillation, and low ejection fraction defines the brand’s role within institutional medicine.
The brand claims that its offerings are adopted by more than 600,000 healthcare providers across hospitals and organized care environments.
However, expanded AI functionality requires an Eko+ subscription after the trial period. Growth and access also remain closely linked to health system partnerships and institutional procurement, including collaborations associated with the NHS. Products are often acquired through organizational purchasing rather than open retail channels. This reinforces clinical credibility but limits flexibility for independent adoption. Consideration should be given to long-term subscription commitments, device compatibility cycles, and reliance on mobile platforms for core functionality.
Contact us at [email protected] or follow @leafsnap on Twitter! View our Privacy Policy.