
Truvaga offers neuro-wellness and neuromodulation devices, which function through vagus nerve stimulation. Such devices may stimulate the vagus nerve, which may help improve sleep quality, lower stress levels, and calm an overactive nervous system.
As per the makers, its VNS offerings come with built-in stimulation parameters aligned with published vagus-nerve stimulation research. The brand further integrates app-enabled control with the Plus model, which may offer convenience and customizable use.
In this review, we explore the brand’s background and how its use of VNS technology may support nervous-system balancing. The review also provides a breakdown of its device models, intended use-cases, and real user experiences with the brand’s services and VNS offerings.
Founded by JP Errico, Truvaga focuses on non-invasive vagus nerve stimulation devices, which may help decrease stress levels and potentially promote a calmer state of mind. The brand’s philosophy and VNS offerings center on the idea that a balanced nervous system may support wellness aspects, such as sleep quality, cognition, and vitality.
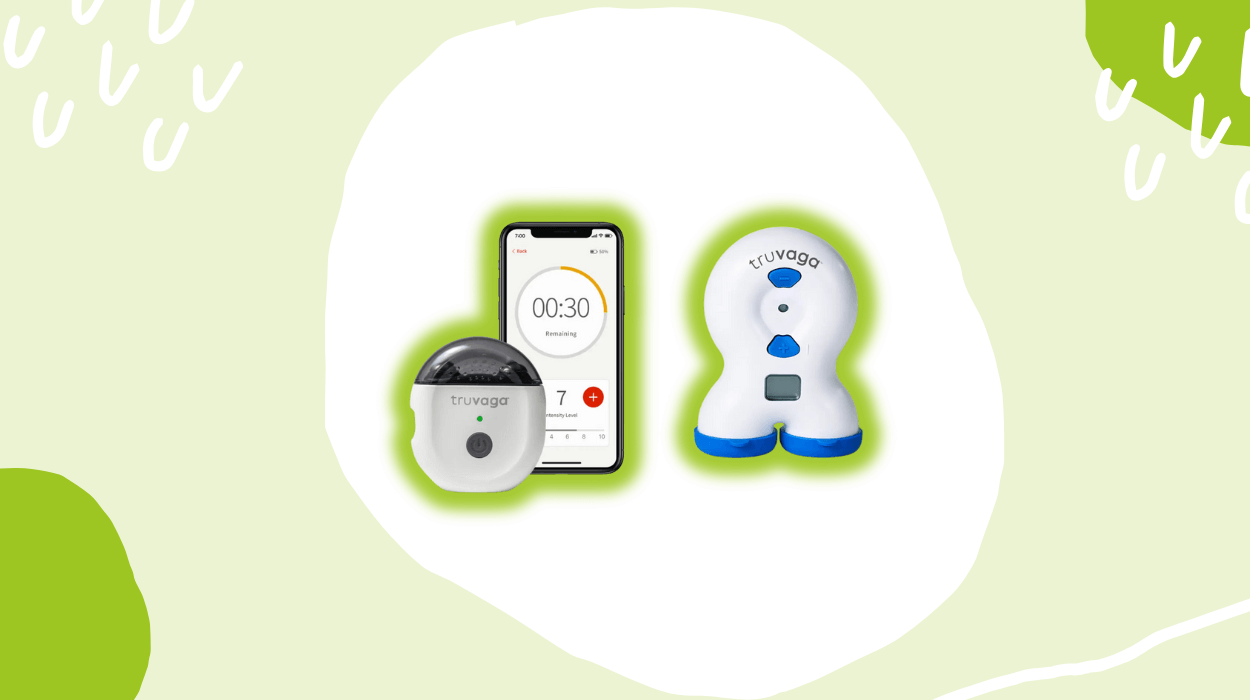
Its lineup includes two core devices, namely the Truvaga 350 and Plus models. Both models use short, two-minute sessions of gentle vagus nerve stimulation to promote relaxation benefits. The 350 model offers preloaded sessions without the need for cords or app connections, keeping the setup simple. Meanwhile, the Plus model introduces features such as unlimited sessions and a rechargeable design.
According to its official site, the brand provides guided training sessions to help you learn how to operate its VNS offerings with more ease. You further get the flexibility to choose a date and a specific time to schedule training from the available slots. Moreover, the Plus model provides access to a dedicated app-based ecosystem, which is available on both Android and IOS devices. Through the app, you can manage your session intensity, view reminders, plan your schedule, and track your progress.

Truvaga Plus utilizes gentle stimulation of the vagus nerve to help calm an overactive nervous system, which may lower stress levels, support digestion, and provide mild mood enhancement benefits. Unlike the standard 350 model, the Plus variant is rechargeable and offers unlimited sessions of 2-minute use, with no requirement for replacement or refill.
As per its official website, the package comes with one unit of the device, a 50 ml bottle of Signaspray electrode spray, a USB-C charging cable, and free access to the Truvaga Plus app.
Operation of the device requires connectivity to the mobile app, which allows you to adjust session settings, track progress, and schedule upcoming sessions. The app can also be integrated with Apple Health to provide a broader look at your wellness data.
Truvaga 350 is a handheld VNS device that could help support relaxation, improve focus, and strengthen the brain–gut connection. According to the official website, the device works without an app, cords, or subscriptions and comes preloaded with 350 sessions—about six months of daily use. Every package includes the device and four 50 ml tubes of SignaGel, a hypoallergenic and residue-free conductivity gel that helps maintain proper contact during each session.
The device delivers mild electrical pulses to the skin over the vagus nerve through transcutaneous vagus nerve stimulation. These low-level impulses activate sensory fibers that connect to the brainstem, including vagal afferent pathways that influence parasympathetic activity. When this area becomes more active, sympathetic activity (responsible for the stress response) typically decreases, while parasympathetic activity increases to support calmness, digestion, and a steady heart rate. This stimulation may also help reduce inflammation and improve sleep quality.
To use the device, locate the vagus nerve by placing two fingers on either side of your neck where the pulse is most noticeable. After identifying the spot, remove the blue caps from the device and apply a pea-sized amount of SignaGel to each electrode. Then place the device on your neck at the identified location. Using the blue button, slowly raise the intensity until you feel a strong yet comfortable tingling sensation. Keep the device in place until it emits two beeps, signaling the end of the session.
The brand claims that its VNS devices, including the 350 and Plus models, are designed around principles observed in clinically studied vagus nerve stimulation. These models use low-level electrical micropulses delivered through metal contact points. When placed on the side of the neck, these pulses target sensory fibers linked to the vagus nerve.
Its 350 model is positioned around basic VNS functionality with no requirement for app login or complicated setup. Meanwhile, the Plus variant features guided sessions through the companion app, preset stimulation patterns, and unlimited 2-minute sessions. The devices focus on controlled electrical stimulation rather than heat, vibration, or invasive methods.
The brand also provides detailed instructions on placement, pathway direction, and expected sensory effects using diagrams and simple explanations, which may help you better understand how the devices work to stimulate the vagus nerve. Such a research-aligned approach might benefit users who prefer technology based on structured concepts studied in clinical environments.
Truvaga designs its VNS devices for simple setup and everyday use, making nervous system support accessible without technical challenges. For example, the 350 model comes with preloaded two-minute sessions and four 50 ml tubes of high-conductivity electrode gel. You simply power it on, place it on the side of your neck, start the session, and remove it when it ends.
The Plus model adds a dedicated app and a rechargeable format but follows the same straightforward handheld placement.
Instructions included in the package focus on easy preparation—ensuring your skin is clean and dry, moving any hair aside, placing the contact area on your neck, and pressing the start button. This minimal setup may be useful if you prefer VNS devices with quick operation and limited setup steps.
Truvaga presents its core VNS offerings, namely the Plus and 350 models, as handheld devices that stimulate the vagus nerve through gentle surface-level electrical pulses.
However, the brand does not provide clinical studies that directly examine how these devices perform in controlled environments. There is no published data on the brand’s official website showcasing any measurable changes in heart-rate variability, cortisol patterns, or autonomic function after using either device.
The brand also does not share independent scientific research comparing Plus or 350 models to medical-grade vagus nerve stimulators or to structured programs like biofeedback, guided neuromodulation, or supervised breathwork. Its VNS offerings include preset stimulation modes and guided session formats, but none of these features have publicly available clinical validation linked directly to the products.
Such a gap in device-specific research could make it difficult to assess what types of outcomes to expect from the brand’s offerings, leaving you with limited objective evidence to determine if they match your needs.
Hoolest and Truvaga both offer non-invasive vagus nerve stimulation devices, which may help support relaxation and nervous system balance.
However, the brands differ in some aspects, including their core philosophy and origins, the direction of their product development, the range of available devices, and how each brand structures its warranty coverage.
Established in 2018 by biomedical engineer Nicholas Hool, the Hoolest brand has developed a patented high-dose vagus nerve stimulation (VNS) technology that uses low-frequency electrical pulses to noninvasively stimulate the auricular branch of the vagus nerve via small electrodes placed just beneath the ear. JP Errico founded Truvage, and electroCore, Inc. develops the brand. While both brands offer VNS-based devices and highlight a science-forward approach to supporting calmness, their product lines branch out in noticeably different ways.
Hoolest’s lineup includes multiple device types, each developed to provide different levels of intensity and target multiple use cases. For instance, it offers the Mini Max PEMF, which is positioned to target the full length of the vagus nerve through powerful electromagnetic fields. The brand’s VeRelief Prime device provides a more compact option and comes with five usage modes alongside two pairs of gel tips. Beyond these, the brand is currently working on Hoolest Pro, which is a hands-free model that pairs Bluetooth and noise cancellation features. However, as of now, the Pro model is only available for pre-booking.
Truvaga keeps its VNS offerings range more limited compared to its competitors like Hoolest. Among its offerings is the 350 model, which is positioned to deliver about 350 sessions of basic VNS functionality. Meanwhile, the brand also offers a Plus model, which covers unlimited use sessions, provides a durable build, and is supported by a dedicated app-based ecosystem.
The warranty structure adds another layer of distinction between the brands. According to its official site, Hoolest provides tiered warranty coverage depending on the device version, with the VeRelief Prime model covered for one year, while the refurbished units have a 90-day limited warranty from the date of delivery.
Moreover, the brand provides an annual membership plan called Hoolest Care Plan, which extends a lifetime warranty for up to three device types, including the Pro, Mini, Prime, and Reset models. This plan covers repair or replacement for defects in materials or workmanship.
On the other hand, Truvaga’s warranty policy terms reflect a simpler approach. The brand provides a limited out-of-box warranty that applies to material defects and focuses on repair, replacement, or refund of eligible products. However, it does not cover issues caused by unauthorized repair attempts, misuse, normal wear and tear, or other external factors.
Pulsetto and Truvaga function in the VNS devices segment, offering a means to tackle an overactive nervous system while promoting calmness and mild mood enhancement benefits. While the brands operate in the same market space, they have some nuances in terms of their pricing structure, range of offerings, quality and safety certifications, and core positioning.
According to its official website, Pulsetto is a wellness technology startup based in Lithuania, founded by Vitalijus Majorovas and Povilas Sabaliauskas. The brand focuses on neuromodulation and VNS technologies, reflecting a European design mindset that emphasizes portability and daily usability.
Meanwhile, Truvaga is a U.S.-based brand that operates under electroCore, Inc., highlighting JP Errico as its founder. ElectroCore’s scientific involvement in vagus nerve research influences the technology and efficacy behind the brand’s VNS offerings.
When comparing product offerings, Pulsetto’s primary lineup centers on two wearable VNS models that intend to stimulate the vagus nerve for calmness via emitting ultra-low radio frequency energy to targeted areas. Among its offerings is the Pulsetto Fit model, which features added padding for a more secure fit, up to 12 days of battery life, and wireless update capabilities. The brand also offers the Pulsetto Lite model, which has a compact design, basic VNS features, and integrates with the Pulsetto app for guided use.
On the other hand, Truvaga’s lineup takes a simpler handheld approach with the 350 model, which requires no app integration and minimal setup hassles. The brand also offers a Plus model, which is equipped with an extended usage lifespan of up to 30,000 sessions, a rechargeable design, and operations with a dedicated app ecosystem.
In terms of pricing structure, there are some differences between the brands. For instance, Pulsetto positions its Fit model in the retail range of $580 and $615, while the Lite model is priced between $450–$500. On the other hand, Truvaga’s pricing falls lower for its entry model, with the 350 variant retailing between $285 and $310, and the Plus model ranging from $485 to $510.
In evaluating Truvaga’s brand reputation, we looked at how the brand presents its background and its ratings across independent review forums. The brand positions itself around supporting nervous system balance through its vagus nerve stimulation devices, emphasizing at-home use and non-invasive technology.
On Trustpilot, the brand holds a 4.1 out of 5 rating, based on a limited number of reviews shared on the platform. Several users described positive experiences related to sleep quality, stress reduction, and mood support, appreciating the brand’s VNS devices. However, one user described not experiencing the claimed relaxation benefits with its devices.
Added insight comes from TenereTeam, where the brand carries a 4.5 out of 5 rating, although from a very small count of publicly available reviews. Taken together, these factors suggest that the brand demonstrates moderate credibility in the VNS devices segment. However, the limited review volume indicates that user experiences may differ across other platforms.
To evaluate Truvaga, we examined verified user feedback shared across Reddit discussions and threads between 2024 and 2025. One user noted that while using the Truvaga 350 model, its suction intensity pulled on a muscle near the windpipe, which led to discomfort such as numbness around the face and neck, a tingling sensation, and pain in the face and jaw.
Other users mentioned that adjusting the device settings and paying attention to placement helped them achieve better relaxation effects. Some users found the brand’s VNS devices helpful in easing mild discomforts linked to migraines or chronic pain. At the same time, a few users shared negative experiences, such as concerns about the device’s durability, difficulties with Android compatibility, and trouble finding a suitable case to store the devices.
Such feedback indicates that experiences with the brand’s offerings vary widely. A few users observed noticeable relaxation benefits, while others encountered potential challenges or side effects.
Truvaga positions itself as a brand that focuses on noninvasive vagus nerve stimulation, with its devices presented to help promote relaxation and support a calmer mental state. It highlights multiple preset modes across the VNS models, availability ot guided training sessions, and an app-based ecosystem with the Plus model.
However, the brand does not provide device-specific clinical studies or controlled research that demonstrates measurable outcomes linked directly to its products. Such absence of product-based data can make it difficult for you to understand how the brand’s VNS offerings compare to medical-grade stimulators or other neuromodulation methods. The brand’s devices do not hold FDA clearance, which may raise potential concerns around their efficacy and safety standards.
Contact us at [email protected] or follow @leafsnap on Twitter! View our Privacy Policy.